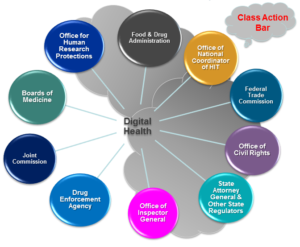
The US healthcare system’s data infrastructure needs an overhaul to prepare for future health crises, streamline patient care, improve data sharing and accessibility among patients, providers and government entities, and move toward the delivery of coordinated care. With insights from leaders from Arcadia, Validic and McDermott, we recently discussed key analyses and updates on the interoperability and application programming interfaces (API) criteria from the 21st Century Cures Act, stakeholder benefits of healthcare data exchange and data submission facilitation for public health purposes. Click here to listen to the webinar recording, and read on for highlights from the program.
To learn more about the “Around the Corner” webinar series and attend an upcoming program, click here.
PROGRAM INSIGHTS
- COVID-19 is reshaping healthcare through technology. Hospitals, clinicians and payors need to use digital health tools to address the challenges of the coronavirus (COVID-19) public health pandemic. How COVID-19 data and health information are captured, and then move through electronic systems, will form the foundation by which digital health tools can become effective in identifying cases, treating them and ensuring favorable outcomes.
- API certification requirements under the 21st Century Cures Act are designed to enhance the accessibility of electronic health information. The 21st Century Cures Act’s purpose is to advance interoperability, address information blocking, support seamless exchange of electronic health information and promote patient access. Putting data from electronic health records (EHRs) into patients’ hands through consumer-facing apps will empower them to understand and take control of their health.
- EHR vendors will be required to offer APIs that comply with the Fast Healthcare Interoperability Resource (FHIR) standard by May 1, 2022. The 21st Century Cures Act Final Rule will require EHR vendors to offer FHIR based APIs that make electronic health information more readily available to third-party applications (apps) of patients’ and providers’ choosing. API standardization will make it easier for third-party developers to build these apps, and for patients and providers wishing to use third-party apps to leverage their electronic health information for various purposes, including health information exchange and population health management.
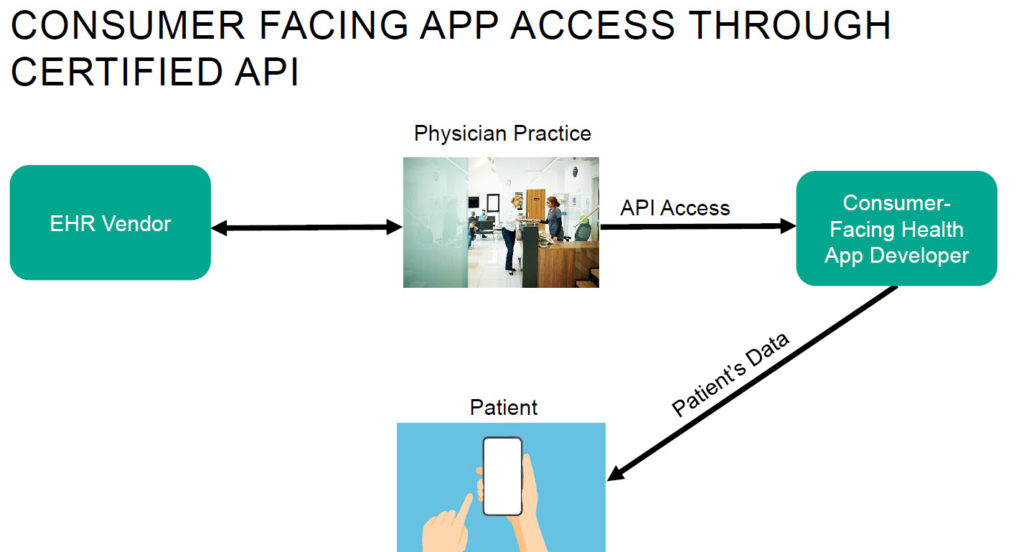
- Interoperability refers to the standards that make it possible for different EHR systems to exchange patient medical records and information between providers. Increased interoperability between EHR systems using harmonized standards allows for a more seamless transfer of patient data between providers. The interoperability requirements in the 21st Century Cures Act have the potential to advance patient access to their data and the use of information among physicians.
- Both providers and patients can drive data exchange. One challenge impacting data exchange between patients and providers is that providers cannot always access or integrate data that patients have created with third-party tools (e.g., fitness trackers). However, there is emerging technology designed to aggregate and standardize consumer-generated health information, [...]
Continue Reading
read more

 Subscribe
Subscribe