The COVID-19 pandemic has catalyzed efforts by health insurers to expand reimbursement for telehealth services and digital health tools, and develop and invest in their own digital health technology. Health insurers, who increasingly play a hybrid role of payor, innovator and provider, have a vested interest in helping consumers manage chronic diseases and engage in preventive care from home, both during the public health emergency and after.
Joined by leaders from Humana, Oscar, and Medorion, we discussed the role of health insurers in the evolving digital health market, reimbursement pathways for digital tools and innovative partnerships between technology companies and health insurers. Click here to listen to the webinar recording, and read on for highlights from the program.
PROGRAM INSIGHTS
- COVID-19 has accelerated the integration of digital health into the traditional health insurance framework. Pre-COVID-19, health insurers were using digital health tools to help their members find providers, access care and manage health conditions. COVID-19 has hastened health plans’ efforts toward vertical integration of digital health technology. Health insurers at the forefront of this effort are focused on creating a consumer-centric, digitally enabled and fully integrated healthcare ecosystem to enhance the member experience, bend the cost curve and carve out an essential (and expanded) role for themselves in the future of healthcare. As consumer behavior continues to change as a result of COVID-19, health insurers will have to be responsive to the way their members are getting care and interacting with the healthcare system.
- Health insurers are uniquely situated to leverage digital health technologies. Data-driven technology is only as good as the data behind it. Due to the critical role health insurers play in paying for healthcare services, they have insight into member patterns of care and utilization that can be used to target interventions, influence member decision-making and improve health. Investments in digital tools and analytics, as well as strategic partnerships with technology companies, will allow for increased leverage of this valuable data, improved integration of member health information and enhanced member engagement.
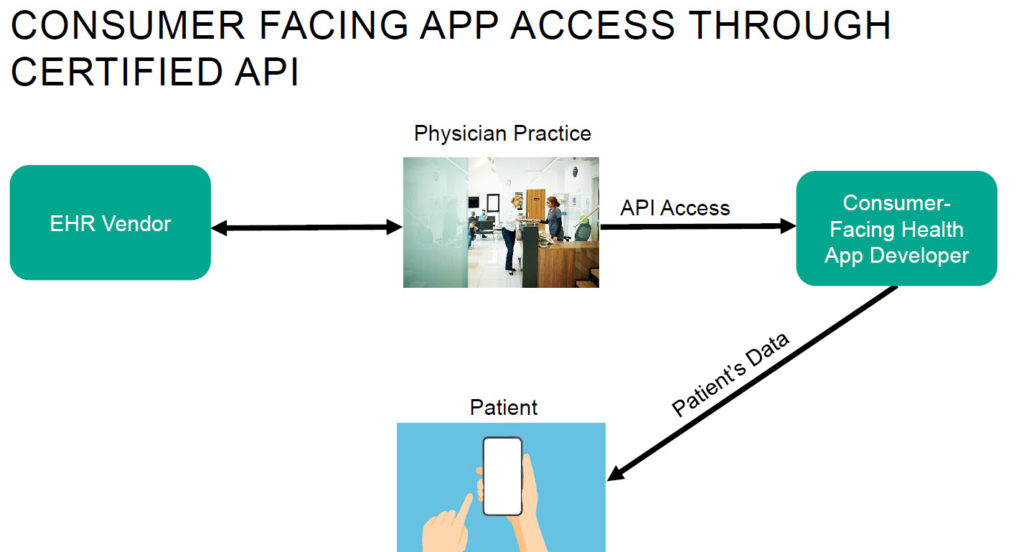
- Interoperability with existing health IT systems is crucial to break down barriers to digital health implementation. Healthcare has been grappling with data interoperability challenges for decades. To scale and make the information from digital tools actionable as part of a larger care plan, digital health platforms must also be interoperable with existing health IT systems. Interoperability will also allow insurers to gather a more complete picture of a member’s longitudinal health data and enable them to better support member health.
- Health insurers and their legal teams will need to remain nimble amidst the rapidly changing regulatory environment. Keeping up with changing regulations during the COVID-19 public health emergency while planning to scale up in terms of technology implementations is a delicate balance. Though federal, state and local agencies appreciate that digital health tools and telemedicine have much potential in terms of patient care, health insurance companies remain vigilant of privacy and security risks and continue to be constrained in their [...]
Continue Reading
read more

 Subscribe
Subscribe