Digital health companies are producing increasingly innovative products at a rapidly accelerating pace, fueled in large part by the expansive healthcare data ecosystem and the data strategies for harnessing the power of that ecosystem. The essential role data strategies play make it imperative to address the data-related legal and regulatory considerations at the outset of the innovation initiative and throughout the development and deployment lifecycle so as to protect your investment in the short and long term.
The Evolution of Digital Health
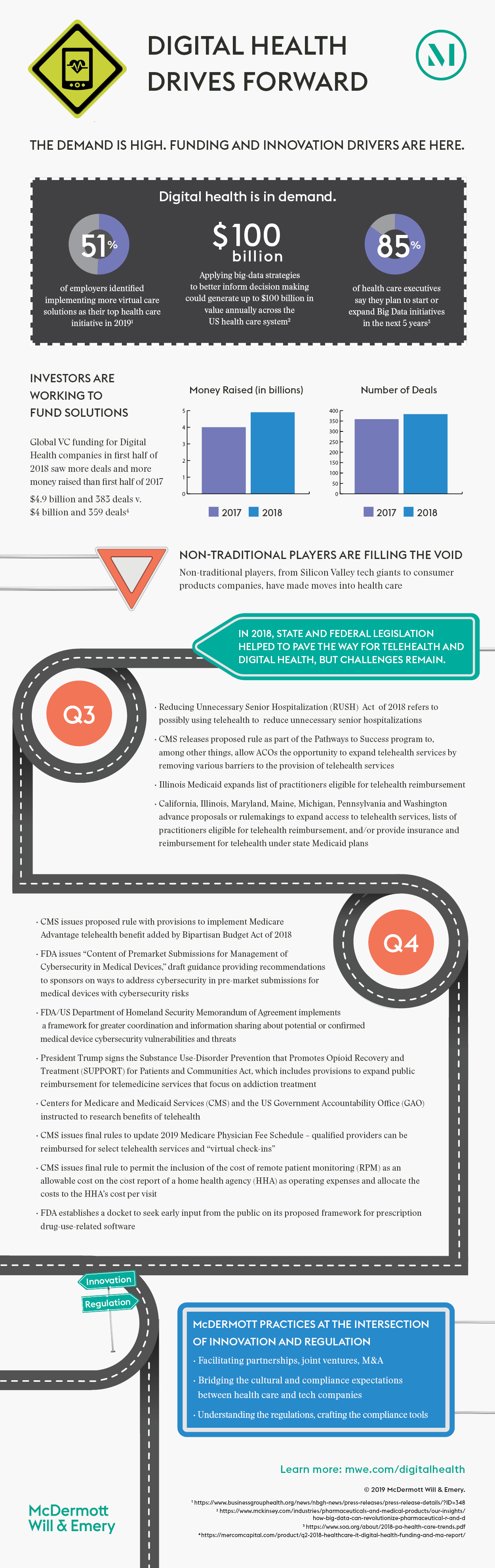
Digital health today consists of four key components: electronic health records, data analytics, telehealth, and patient and consumer engagement tools. Electronic health records were most likely first, followed very closely by data analytics. Then telehealth deployment rapidly increased in response to both demand by patients and providers, the improved care delivery and access it offers, and more recently, the expanded reimbursement for telehealth solutions. Each component of digital health was developed somewhat independently, but they have now converged and are interrelated, integral parts of the overall digital health ecosystem.
The patient and consumer engagement dimension of digital health has exploded over the last five years. This is due, in large part, to consumer and patient demand for greater engagement in the management of their healthcare, as well as the entry of disruptors, such as technology service providers, e-commerce companies, consumer products companies and entrepreneurs. At this point in the evolution of the digital health landscape, the patient and consumer engagement tool dimension pulls in all other key components and no digital health consumer engagement tool is complete without the full package.
Data Strategies and Collaborations as Key Innovation Ingredients
No digital health initiative can be developed, pursued or commercialized without data. But the world of data aggregation and analytics has also changed significantly and become immensely complex in recent years. Digital health innovation is no longer working exclusively within the friendly confines of the electronic health record and the carefully regulated, controlled and structured data it holds. Today, digital health innovation relies on massive amounts of data in a variety of types, in various forms, from a wide variety of sources, and through a wide variety of tools, including patient and consumer wearables and mobile devices.
read more

 Subscribe
Subscribe